Erik Engelson, President & CEO | Bioengineering Alumnus '84

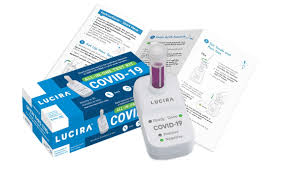 The U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the first COVID-19 diagnostic test for self-testing at home and that provides rapid results. The Lucira COVID-19 All-In-One Test Kit is a molecular (real-time loop mediated amplification reaction) single use test that is intended to detect the novel coronavirus SARS-CoV-2 that causes COVID-19. >> read FDA News Release
The U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the first COVID-19 diagnostic test for self-testing at home and that provides rapid results. The Lucira COVID-19 All-In-One Test Kit is a molecular (real-time loop mediated amplification reaction) single use test that is intended to detect the novel coronavirus SARS-CoV-2 that causes COVID-19. >> read FDA News Release
Engelson earned his bachelor’s degree in microbiology from UC San Diego, and then a master’s in bioengineering from the Jacobs School of Engineering at UC San Diego. Engelson is a Trustee Emeritus of the UC San Diego Foundation; Initial Chairman of the Board of Trustees of UC San Diego’s Bioengineering Department; a member of the UC San Diego Division of Biological Sciences Dean’s Leadership Council; and an elected fellow of the American Institute for Medical and Biological Engineering (AIMBE).
